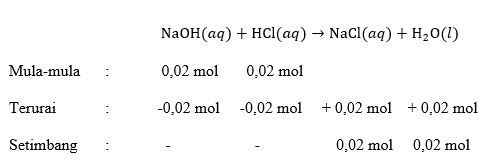Iklan
Pertanyaan
Sebanyak 100 mL NaOH 0,2 M dan 100 mL HCI 0,2 M direaksikan ke dalam kaiorimeter. Suhu awal rata-rata kedua Iarutan 26 ∘ C dan setelah reaksi suhunya menjadi 32 ∘ C . Apabila kalor jenis larutan dan massa jenis Iarutan = 1 g mL − 1 , reaksi termokimia yang paling tepat adalah ....
Sebanyak 100 mL NaOH 0,2 M dan 100 mL HCI 0,2 M direaksikan ke dalam kaiorimeter. Suhu awal rata-rata kedua Iarutan dan setelah reaksi suhunya menjadi . Apabila kalor jenis larutan dan massa jenis Iarutan = , reaksi termokimia yang paling tepat adalah ....
Iklan
IS
I. Solichah
Master Teacher
Jawaban terverifikasi
25
0.0 (0 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia