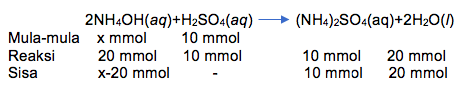Iklan
Pertanyaan
Ke dalam 100 mL larutan H 2 SO 4 ( pH = 0 , 6990 ) dialirkan gas amonia hingga pH menjadi 9 + lo g 2 . Berapa mL volume gas NH 3 yang dialirkan ke dalam larutan tersebut diukur pada 0 ∘ C dan 76 cmHg ? ( K b NH 3 = 2 × 1 0 − 5 )
Ke dalam 100 mL larutan dialirkan gas amonia hingga pH menjadi . Berapa mL volume gas yang dialirkan ke dalam larutan tersebut diukur pada ?
Iklan
LA
L. Avicenna
Master Teacher
Mahasiswa/Alumni Institut Teknologi Bandung
Jawaban terverifikasi
1
4.6 (3 rating)
a
aldi
Pembahasan lengkap banget Bantu banget Mudah dimengerti
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia