Iklan
Pertanyaan
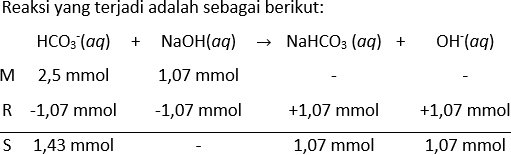
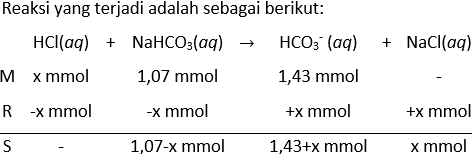
Suatu larutan penyangga dibuat melalui pencampuran 50,0 mL larutan natrium bikarbonat ( NaHCO 3 ) 0,050 M dan 10,7 mL larutan NaOH 0,10 M. ( K a HCO 3 − = 4 , 69 × 1 0 − 11 ) . Berapakah pH larutan penyangga? Berapa gram HClyang harus ditambahkan ke dalam 25,0 mL larutan penyangga ini agar pH turun sebesar 0.07 satuan?
Suatu larutan penyangga dibuat melalui pencampuran 50,0 mL larutan natrium bikarbonat 0,050 M dan 10,7 mL larutan NaOH 0,10 M. .
- Berapakah pH larutan penyangga?
- Berapa gram HCl yang harus ditambahkan ke dalam 25,0 mL larutan penyangga ini agar pH turun sebesar 0.07 satuan?
Iklan
Q'
Q. 'Ainillana
Master Teacher
Mahasiswa/Alumni Universitas Negeri Yogyakarta
Jawaban terverifikasi
1
0.0 (0 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia