Iklan
Pertanyaan
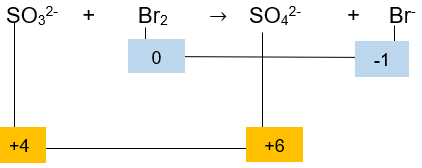
Setarakan reaksi berikut dengan metode biloks atau ion elektron! SO 3 2 − + Br 2 → SO 4 2 − + 2 Br − (asam)
Setarakan reaksi berikut dengan metode biloks atau ion elektron!
(asam)
Iklan
IY
I. Yassa
Master Teacher
Jawaban terverifikasi
1
5.0 (2 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia