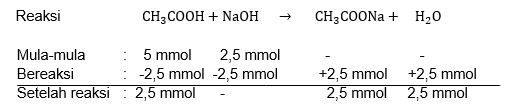Iklan
Pertanyaan
Pada titrasi 50 mL CH 3 COOH 0,1 M ( Ka CH 3 COOH = 1 , 8 x 1 0 − 5 ) dengan NaOH 0,1 M. Tentukan nilai pH larutan setelah penambahan NaOH sebesar: a. 0 mL b. 25 mL
Pada titrasi 50 mL 0,1 M dengan NaOH 0,1 M. Tentukan nilai pH larutan setelah penambahan NaOH sebesar:
a. 0 mL
b. 25 mL
Iklan
IS
I. Solichah
Master Teacher
Jawaban terverifikasi
13
4.1 (10 rating)
NH
Nazwa Hatwan
Pembahasan lengkap banget Ini yang aku cari! Mudah dimengerti Makasih ❤️ Bantu banget
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia