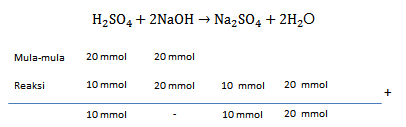Iklan
Pertanyaan
Kelvin melakukan percobaan untuk menentukan pH suatu larutan. Kemudian dia mencampurkan larutan asam sulfat 0,2 M sebanyak 100 mL dan larutan NaOH 100 mL 0,2 M. Berapakah pH larutan sebelum di campur dan sesudah dicampur?
Kelvin melakukan percobaan untuk menentukan pH suatu larutan. Kemudian dia mencampurkan larutan asam sulfat 0,2 M sebanyak 100 mL dan larutan NaOH 100 mL 0,2 M. Berapakah pH larutan sebelum di campur dan sesudah dicampur?
Iklan
FK
F. Krisna
Master Teacher
Mahasiswa/Alumni Institut Pertanian Bogor
Jawaban terverifikasi
3
5.0 (1 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia