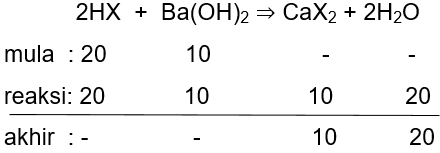Iklan
Pertanyaan
Sebanyak 50 mL larutan HX 0,4 M direaksikan dengan 50 mL larutan Ba(OH) 2 0,2 M. Maka pH larutan tersebut adalah ... (K a = 2 × 1 0 − 5 )
Sebanyak 50 mL larutan HX 0,4 M direaksikan dengan 50 mL larutan Ba(OH)2 0,2 M. Maka pH larutan tersebut adalah ... (Ka=)
Iklan
AN
A. Nurul
Master Teacher
Mahasiswa/Alumni UIN Syarif Hidayatullah Jakarta
Jawaban terverifikasi
3
5.0 (1 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia