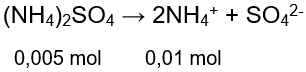Iklan
Iklan
Pertanyaan
Sebanyak 50 ml ( NH 4 ) 2 SO 4 0,1 M dicampur dengan 50 ml larutan NH 3 0,1 M ( K b = 1 0 − 5 ) .Tentukan p H campuran yang terjadi!
Sebanyak 50 ml 0,1 M dicampur dengan 50 ml larutan 0,1 M . Tentukan pH campuran yang terjadi!
Iklan
ED
E. Dwihermiati
Master Teacher
Mahasiswa/Alumni Universitas Pendidikan Indonesia
Jawaban terverifikasi
35
5.0 (1 rating)
NN
NEINTHORIYADI NEINTHORIYADI
Bantu banget Makasih ❤️
Iklan
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2024 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia