Iklan
Pertanyaan
Perhatikan tabel larutan penyangga beserta komposisinya berikut! Diketahui Ka HNO 2 = 4 , 5 × 1 0 − 4 . Urutan harga pH mulai dari yang terkecil hingga terbesar adalah ....
Perhatikan tabel larutan penyangga beserta komposisinya berikut!
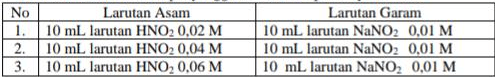
Diketahui . Urutan harga pH mulai dari yang terkecil hingga terbesar adalah ....
Iklan
MJ
M. Juliana
Master Teacher
Mahasiswa/Alumni Universitas Negeri Medan
Jawaban terverifikasi
2
5.0 (2 rating)
RH
Ridha Harahap
Pembahasan lengkap banget
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia















