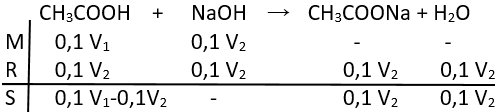Iklan
Pertanyaan
Perbandingan volume larutan CH 3 COOH 0,1 M dan NaOH 0,1 M yang harus dicampurkan untuk membuat larutan penyangga dengan pH = 6 adalah ... (K a = 10 -5 )
Perbandingan volume larutan CH3COOH 0,1 M dan NaOH 0,1 M yang harus dicampurkan untuk membuat larutan penyangga dengan pH = 6 adalah ... (Ka = 10-5)
Iklan
AN
A. Nurul
Master Teacher
Mahasiswa/Alumni UIN Syarif Hidayatullah Jakarta
Jawaban terverifikasi
17
4.4 (38 rating)
K
Kurniawan
Makasih ❤️
in
icalima niza
Pembahasan tidak menjawab soal Ini yang aku cari!
Kf
Kanaya fitri anita
Makasih ❤️
KD
Khusnul Diahayu Sepriani
Makasih ❤️
R
Rara
Makasih ❤️ Ini yang aku cari!
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia