Pertanyaan
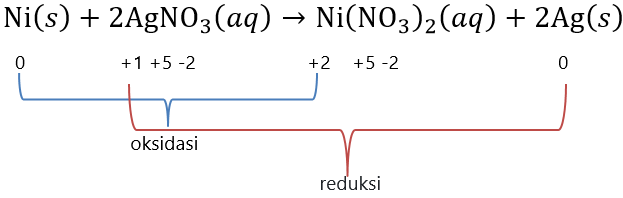
Pada reaksi: Ni ( s ) + 2 AgNO 3 ( a q ) → Ni ( NO 3 ) 2 ( a q ) + 2 Ag ( s ) Menurut reaksi diatas bagan sel volta dapat dituliskan dengan ...
Pada reaksi:
Menurut reaksi diatas bagan sel volta dapat dituliskan dengan ...


Belajar bareng Champions
Brain Academy Champions
Hanya di Brain Academy
Habis dalam
00
:
00
:
16
:
58
AN
A. Nurul
Master Teacher
Mahasiswa/Alumni UIN Syarif Hidayatullah Jakarta
Jawaban terverifikasi
16
5.0 (1 rating)
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2024 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia