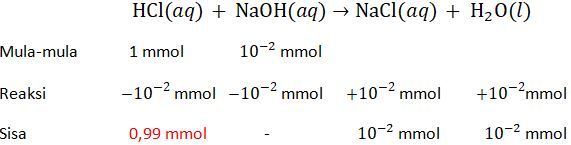Iklan
Pertanyaan
Jika 100 mL larutan asam klorida dengan pH = 2 dicampurkan pada 100 mL larutan natrium hidroksida dengan pH = 10, akan diperoleh larutan dengan ....
Jika 100 mL larutan asam klorida dengan pH = 2 dicampurkan pada 100 mL larutan natrium hidroksida dengan pH = 10, akan diperoleh larutan dengan ....
pH = 3
pH = 6
2 < pH < 6
3 < pH < 6
6 < pH < 1
Iklan
DH
D. Hidayat
Master Teacher
Mahasiswa/Alumni Universitas Sebelas Maret
Jawaban terverifikasi
17
4.9 (11 rating)
ry
rahma yanti
Makasih ❤️ Pembahasan lengkap banget Mudah dimengerti Bantu banget Ini yang aku cari!
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2024 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia