Iklan
Pertanyaan
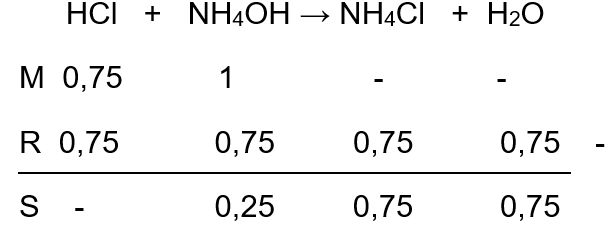
Gas HCl murni, 18 mL dan gas NH 3 murni 24 mL dilarutkan ke dalam 250 ml air sehingga seluruh gas larut dan tidak merubah volume air. Tekanan gas - gas semula 76 cmHg dan temperaturnya 27°C. Kalau tetapan (konstanta) gas ideal adalah R=0,08 L atm mol -1 K -1 , Kb NH 4 OH = 1 x 10 -5 , log 2 = 0,3; log 3 = 0,47 dan log 5 = 0,70 maka pH larutan tersebut adalah ...
Gas HCl murni, 18 mL dan gas NH3 murni 24 mL dilarutkan ke dalam 250 ml air sehingga seluruh gas larut dan tidak merubah volume air. Tekanan gas - gas semula 76 cmHg dan temperaturnya 27°C. Kalau tetapan (konstanta) gas ideal adalah R=0,08 L atm mol-1K-1, Kb NH4OH = 1 x 10-5, log 2 = 0,3; log 3 = 0,47 dan log 5 = 0,70 maka pH larutan tersebut adalah ...
Iklan
A. Nurul
Master Teacher
Mahasiswa/Alumni UIN Syarif Hidayatullah Jakarta
13
4.5 (8 rating)
Aldi Kusuma putra
Makasih ❤️
meita nawangsari
Pembahasan terpotong
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia