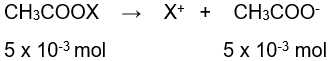Iklan
Pertanyaan
Dalam laboratorium kimia, larutan penyangga bisa dibuat dengan cara mencampurkan asam lemah dengan garamnya atau basa lemah dengan garamnya. Seorang laboran kimia ingin membuat larutan penyangga dengan p H 5 - log 2. Dia mencampurkan sebanyak 0,49 gram garam CH 3 COOX ke dalam 200 mL larutan CH 3 COOH 0,025 M ( K a CH 3 COOH = 2 × 1 0 − 5 ) . Unsur X yang terkandung dalam garam tersebut adalah ... ( A r : C = 12, H = 1, O = 16, Mg = 24, Ca = 40, K = 39, Na = 23, Ba = 137)
Dalam laboratorium kimia, larutan penyangga bisa dibuat dengan cara mencampurkan asam lemah dengan garamnya atau basa lemah dengan garamnya. Seorang laboran kimia ingin membuat larutan penyangga dengan pH 5 - log 2. Dia mencampurkan sebanyak 0,49 gram garam ke dalam 200 mL larutan 0,025 M . Unsur X yang terkandung dalam garam tersebut adalah ... (: C = 12, H = 1, O = 16, Mg = 24, Ca = 40, K = 39, Na = 23, Ba = 137)
Iklan
IS
I. Solichah
Master Teacher
Jawaban terverifikasi
2
0.0 (0 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia