Iklan
Pertanyaan
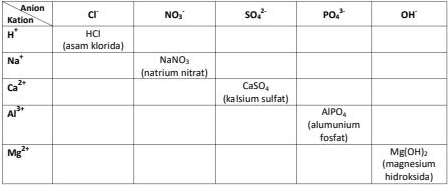
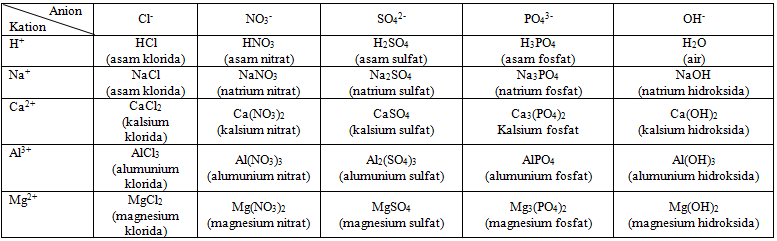
Lengkapilah tabel di bawah ini dengan cara menggabungkan antara kation dan anion serta tuliskanlah nama senyawa sesuai dengan tata nama senyawa IUPAC. RUMUS : Kation + Anion
Lengkapilah tabel di bawah ini dengan cara menggabungkan antara kation dan anion serta tuliskanlah nama senyawa sesuai dengan tata nama senyawa IUPAC.
RUMUS : Kation + Anion
Iklan
IS
I. Solichah
Master Teacher
Jawaban terverifikasi
37
3.5 (4 rating)
R
Rosta
Ini yang aku cari! Mudah dimengerti
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia



 .
.











