Iklan
Pertanyaan
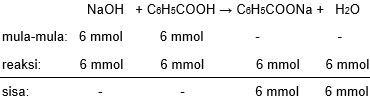
Larutan NaOH dengan volume 100 mL mempunyai pH sebesar 12 + log 6 dicampurkan ke dalam 400 mL larutan CH 3 COOH dengan pH sebesar 3,5 - log 3. Jika harga K a CH 3 COOH = 6 , 0 × 1 0 − 5 , hitung pH campuran yang terbentuk!
Larutan dengan volume 100 mL mempunyai pH sebesar 12 + log 6 dicampurkan ke dalam 400 mL larutan dengan pH sebesar 3,5 - log 3. Jika harga = , hitung pH campuran yang terbentuk!
Iklan
DV
D. Vitama
Master Teacher
Mahasiswa/Alumni Universitas Islam Negeri Sunan Kalijaga
Jawaban terverifikasi
4
0.0 (0 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia