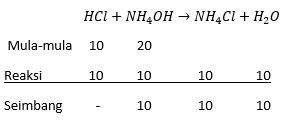Iklan
Pertanyaan
Jika 100 ml HCl 0,1 M direaksikan dengan 200 ml NH 4 OH 0,1 M membentuk reaksi di bawah ini : HCl + NH 4 OH → NH 4 Cl + H 2 O pH larutan di atas adalah .... ( Kb = 1 × 1 0 − 5 )
Jika 100 ml 0,1 M direaksikan dengan 200 ml 0,1 M membentuk reaksi di bawah ini :
pH larutan di atas adalah ....
Iklan
DZ
D. Zharva
Master Teacher
Jawaban terverifikasi
23
5.0 (2 rating)
Iklan
Pertanyaan serupa
Tanya ke AiRIS
Yuk, cobain chat dan belajar bareng AiRIS, teman pintarmu!
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia