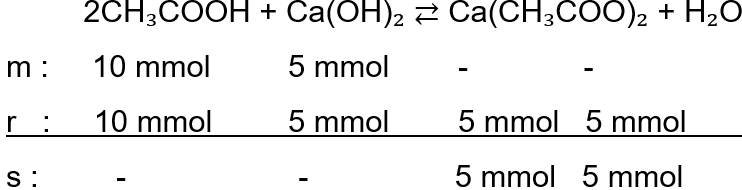Iklan
Iklan
Pertanyaan
Hitunglah pH campuran berikut ! 50 mL larutan CH 3 COOH 0,2 M dengan 50 mL larutan Ca(OH) 2 0,1 M (Ka = 10 -5 )
Hitunglah pH campuran berikut !
50 mL larutan CH3COOH 0,2 M dengan 50 mL larutan Ca(OH)2 0,1 M (Ka = 10-5)
Iklan
RH
R. Harmiasri
Master Teacher
Mahasiswa/Alumni Universitas Negeri Semarang
Jawaban terverifikasi
93
3.4 (5 rating)
DF
Dina Fitriyana Puspita
Pembahasan lengkap banget Makasih ❤️
XF
Xy Fatt
Jawaban tidak sesuai
IN
I Nym Yudha Bayu Dharmika
Pembahasan terpotong
Iklan
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2024 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia