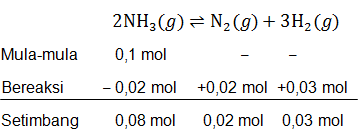Iklan
Pertanyaan
Dalam suatu wadah yang volumenya 2 liter dimasukkan 0,1 mol gas NH 3 menurut reaksi : 2 NH 3 ( g ) ⇌ N 2 ( g ) + 3 H 2 ( g ) . Jika gas N 2 yang terbentuk adalah 0,02 mol dan gas H 2 adalah 0,03 mol. Tentukan nilai
Dalam suatu wadah yang volumenya 2 liter dimasukkan 0,1 mol gas menurut reaksi :
. Jika gas yang terbentuk adalah 0,02 mol dan gas adalah 0,03 mol. Tentukan nilai
...
...
Iklan
NP
N. Puspita
Master Teacher
Jawaban terverifikasi
1
0.0 (0 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia