Iklan
Pertanyaan
Dalam suatu eksperimen dilakukan reaksi ion amonium dan ion nitrit dalam air membentuk gas nitrogen sebagai berikut. NH 4 + ( a q ) + NO 2 – ( a q ) → N 2 ( g ) + 2 H 2 O ( l ) Data eksperimen dengan kemolaran diubah-ubah diperoleh data sebagai berikut. Dari data eksperimen tersebut tentukan orde reaksi dan persamaan laju reaksi.
Dalam suatu eksperimen dilakukan reaksi ion amonium dan ion nitrit dalam air membentuk gas nitrogen sebagai berikut.
Data eksperimen dengan kemolaran diubah-ubah diperoleh data sebagai berikut.
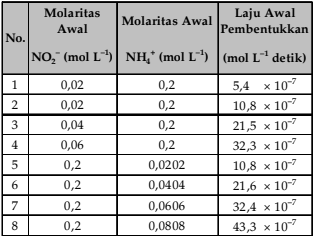
Dari data eksperimen tersebut tentukan orde reaksi dan persamaan laju reaksi.
Iklan
IS
I. Solichah
Master Teacher
Jawaban terverifikasi
1
5.0 (1 rating)
c
cleo
Pembahasan lengkap banget Ini yang aku cari! Bantu banget Makasih ❤️ Mudah dimengerti
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia















