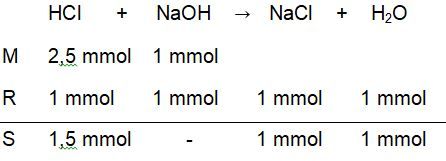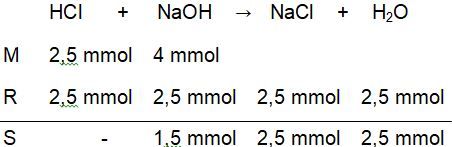Iklan
Pertanyaan
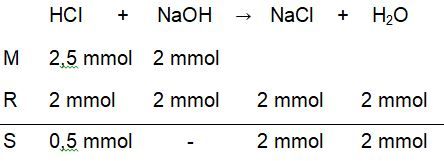
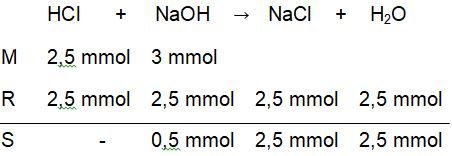
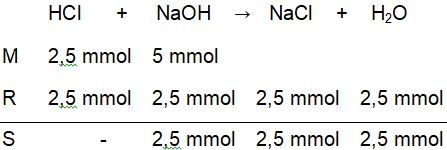
Titrasi larutan 25 ml HCl 0,1 M dengan 50 ml NaOH 0,1 M dengan indikator phenolftalein (PP).Hitunglah perubahan pH pada proses titrasi tersebut mulai dari penambahan 0 ml sampai dengan 50 ml .
Titrasi larutan 25 ml 0,1 M dengan 50 ml 0,1 M dengan indikator phenolftalein (PP). Hitunglah perubahan pH pada proses titrasi tersebut mulai dari penambahan 0 ml sampai dengan 50 ml .
...
...
Iklan
RA
R. Anisa
Master Teacher
Mahasiswa/Alumni Universitas Negeri Semarang
Jawaban terverifikasi
1
0.0 (0 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia