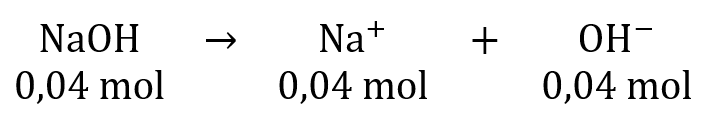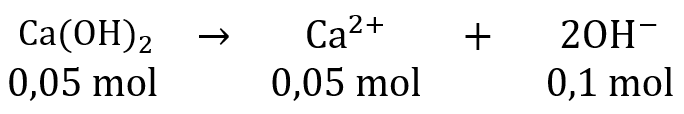Iklan
Pertanyaan
Terdapat 200 mL larutan NaOH 0,2 M,jika kedalamnya ditambah dengan 3,7 gr Ca ( OH ) 2 maka pH campurannya adalah....
Terdapat 200 mL larutan 0,2 M, jika kedalamnya ditambah dengan 3,7 gr maka pH campurannya adalah....
Iklan
SL
S. Lubis
Master Teacher
Jawaban terverifikasi
1
5.0 (1 rating)
n
nblya
Mudah dimengerti
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia