Iklan
Pertanyaan
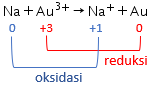
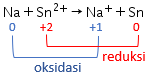
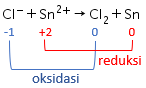
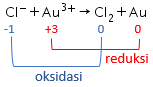
Perhatikan data potensial reduksi standar berikut! Au 3 + + 3 e − Cl 2 + 2 e − Sn 2 + + 2 e − Na + + e − → → → → Au E 0 = + 1 , 42 V 2 Cl − E 0 = + 1 , 36 V Sn E 0 = − 0 , 14 V Na E 0 = − 2 , 71 V Reaksi berikut yang berlangsung tidak spontan adalah ....
Perhatikan data potensial reduksi standar berikut!
Reaksi berikut yang berlangsung tidak spontan adalah ....
Iklan
SI
S. Ibabas
Master Teacher
Jawaban terverifikasi
2
5.0 (3 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2026 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia