Iklan
Pertanyaan
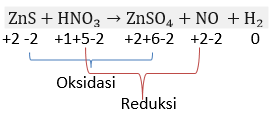
Kepingan seng sulfida bereaksi dengan larutan asam nitrat menghasilkan larutan seng sulfat, gas nitrogen monoksida dan air. Tulis persamaan reaksinya dan setarakan!
Kepingan seng sulfida bereaksi dengan larutan asam nitrat menghasilkan larutan seng sulfat, gas nitrogen monoksida dan air. Tulis persamaan reaksinya dan setarakan!
Iklan
AN
A. Nurul
Master Teacher
Mahasiswa/Alumni UIN Syarif Hidayatullah Jakarta
Jawaban terverifikasi
30
5.0 (2 rating)
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2025 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia