Jasmine N
13 Oktober 2025 15:23
Iklan
Jasmine N
13 Oktober 2025 15:23
Pertanyaan
Setarakan persamaan reaksi berikut: 1. H₂ + O₂ → H₂O 2. H₂ + Cl₂ → HCl 3. Na + O₂ → Na₂O 4. Mg + Cl₂ → MgCl₂ 5. CO + O₂ → CO₂ 6. SO₂ + O₂ → SO₃ 7. Mg + HCl → MgCl₂ + H₂ 8. Al + HCl → AlCl₃ + H₂ 9. NaOH + H₂SO₄ → Na₂SO₄ + H₂O 10. Ca(OH)₂ + HNO₃ → Ca(NO₃)₂ + H₂O 11. CO₂ + Ca(OH)₂ → CaCO₃ + H₂O 12. NaOH + H₃PO₄ → Na₃PO₄ + H₂O 13. Al(OH)₃ + H₂SO₄ → Al₂(SO₄)₃ + H₂O 14. Al(OH)₃ + HNO₃ → Al(NO₃)₃ + H₂O 15. Cl₂ + KOH → KCl + KClO + H₂O 16. Cl₂ + KOH → KCl + KClO₃ + H₂O
Setarakan persamaan reaksi berikut:
1. H₂ + O₂ → H₂O
2. H₂ + Cl₂ → HCl
3. Na + O₂ → Na₂O
4. Mg + Cl₂ → MgCl₂
5. CO + O₂ → CO₂
6. SO₂ + O₂ → SO₃
7. Mg + HCl → MgCl₂ + H₂
8. Al + HCl → AlCl₃ + H₂
9. NaOH + H₂SO₄ → Na₂SO₄ + H₂O
10. Ca(OH)₂ + HNO₃ → Ca(NO₃)₂ + H₂O
11. CO₂ + Ca(OH)₂ → CaCO₃ + H₂O
12. NaOH + H₃PO₄ → Na₃PO₄ + H₂O
13. Al(OH)₃ + H₂SO₄ → Al₂(SO₄)₃ + H₂O
14. Al(OH)₃ + HNO₃ → Al(NO₃)₃ + H₂O
15. Cl₂ + KOH → KCl + KClO + H₂O
16. Cl₂ + KOH → KCl + KClO₃ + H₂O
1
2
Iklan
Matteo A
15 Oktober 2025 02:06
<p>1. <strong>2</strong>H₂ + O₂ → <strong>2</strong>H₂O</p><p><br>2. H₂ + Cl₂ → <strong>2</strong>HCl</p><p><br>3. <strong>4</strong>Na + O₂ → 2Na₂O</p><p><br>4. Mg + Cl₂ → MgCl₂</p><p><br>5. <strong>2</strong>CO + O₂ → <strong>2</strong>CO₂</p><p><br>6. <strong>2</strong>SO₂ + O₂ → <strong>2</strong>SO₃</p><p><br>7. Mg + <strong>2</strong>HCl → MgCl₂ + H₂</p><p><br>8. <strong>2</strong>Al + <strong>6</strong>HCl → <strong>2</strong>AlCl₃ + <strong>3</strong>H₂</p><p><br>9. <strong>2</strong>NaOH + H₂SO₄ → Na₂SO₄ + <strong>2</strong>H₂O</p><p><br>10. Ca(OH)₂ + <strong>2</strong>HNO₃ → Ca(NO₃)₂ + <strong>2</strong>H₂O</p><p><br>11. CO₂ + Ca(OH)₂ → CaCO₃ + H₂O</p><p><br>12. <strong>3</strong>NaOH + H₃PO₄ → Na₃PO₄ + <strong>3</strong>H₂O</p><p><br>13.<strong>2</strong>Al(OH)₃ + <strong>3</strong>H₂SO₄ → Al₂(SO₄)₃ + <strong>6</strong>H₂O</p><p><br>14. Al(OH)₃ + <strong>3</strong>HNO₃ → Al(NO₃)₃ + <strong>3</strong>H₂O</p><p><br>15. Cl₂ + <strong>2</strong>KOH → KCl + KClO + H₂O</p><p><br>16. <strong>2</strong>Cl₂ + <strong>4</strong>KOH → <strong>3</strong>KCl + KClO₃ + H₂O</p>
1. 2H₂ + O₂ → 2H₂O
2. H₂ + Cl₂ → 2HCl
3. 4Na + O₂ → 2Na₂O
4. Mg + Cl₂ → MgCl₂
5. 2CO + O₂ → 2CO₂
6. 2SO₂ + O₂ → 2SO₃
7. Mg + 2HCl → MgCl₂ + H₂
8. 2Al + 6HCl → 2AlCl₃ + 3H₂
9. 2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
10. Ca(OH)₂ + 2HNO₃ → Ca(NO₃)₂ + 2H₂O
11. CO₂ + Ca(OH)₂ → CaCO₃ + H₂O
12. 3NaOH + H₃PO₄ → Na₃PO₄ + 3H₂O
13.2Al(OH)₃ + 3H₂SO₄ → Al₂(SO₄)₃ + 6H₂O
14. Al(OH)₃ + 3HNO₃ → Al(NO₃)₃ + 3H₂O
15. Cl₂ + 2KOH → KCl + KClO + H₂O
16. 2Cl₂ + 4KOH → 3KCl + KClO₃ + H₂O
· 0.0 (0)
Iklan
Yoel F
18 Oktober 2025 13:58
<p>Jawaban Kimia</p><p> </p>
Jawaban Kimia
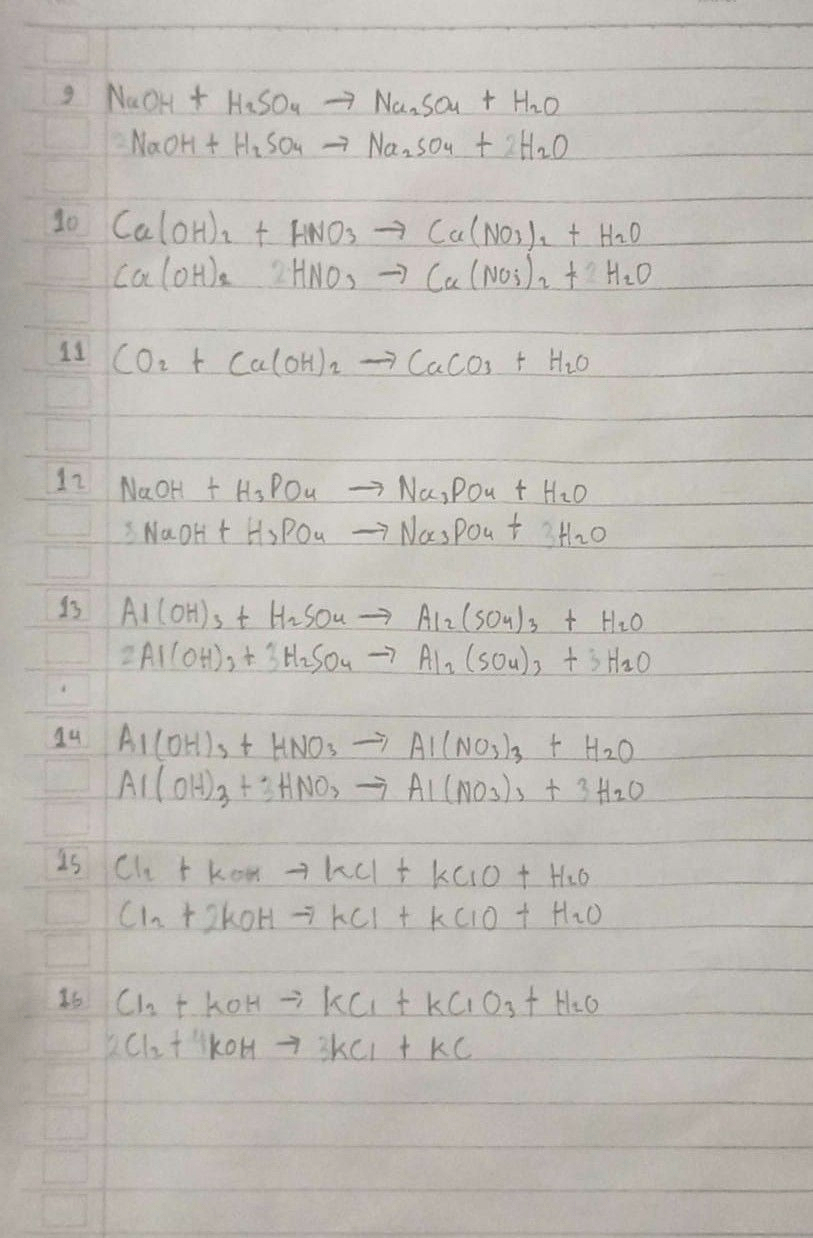
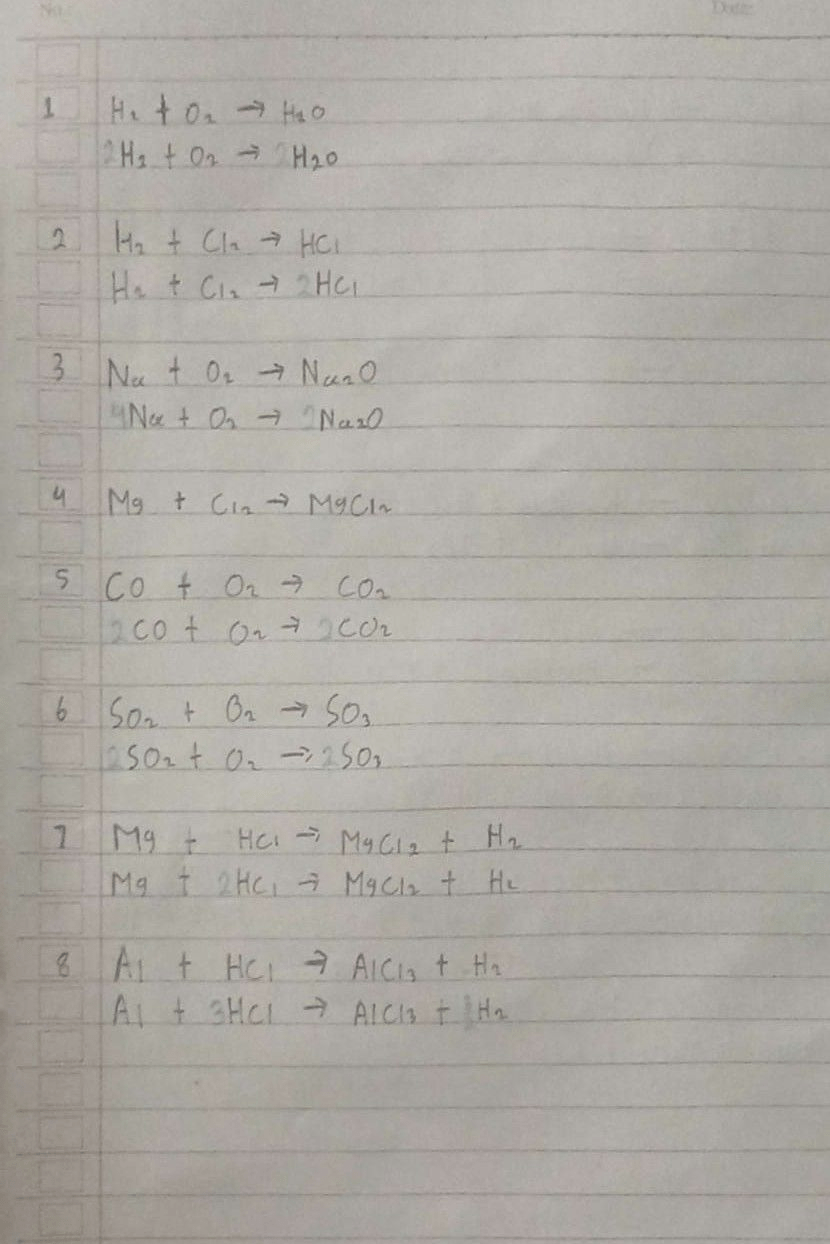
· 0.0 (0)
Mau jawaban yang terverifikasi?
Tanya ke AiRIS
Yuk, cobain chat dan belajar bareng AiRIS, teman pintarmu!

LATIHAN SOAL GRATIS!
Drill Soal
Latihan soal sesuai topik yang kamu mau untuk persiapan ujian


Perdalam pemahamanmu bersama Master Teacher
di sesi Live Teaching, GRATIS!



