Iklan
Iklan
Pertanyaan
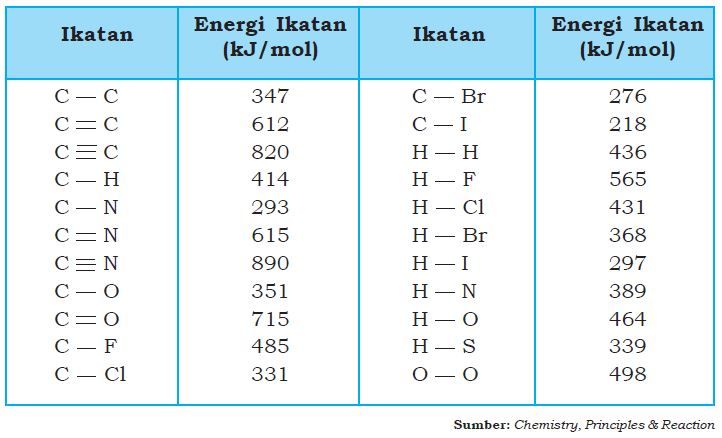
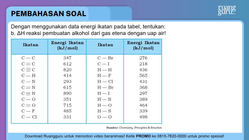
Dengan menggunakan data energi ikatan pada tabel, tentukan: b. △ H reaksi pembuatan alkohol dari gas etena dengan uap air!
Dengan menggunakan data energi ikatan pada tabel, tentukan:
b. reaksi pembuatan alkohol dari gas etena dengan uap air!
Iklan
NP
N. Puspita
Master Teacher
Jawaban terverifikasi
6
0.0 (0 rating)
Iklan
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2024 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia