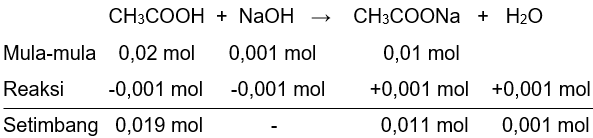Iklan
Iklan
Pertanyaan
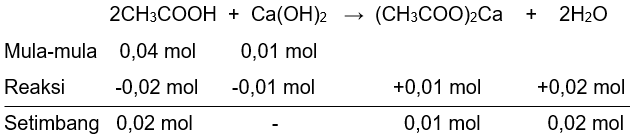
400 mL larutan CH 3 COOH 0,1 M direaksikan dengan 100 mL larutan Ca ( OH ) 2 0,1 M. Tentukan: ( K a = 1 0 − 5 ) a. pH larutan b. pH larutan jika ditambahkan 0,001 mol HCl c.pH larutan jika ditambahkan 0,001 mol NaOH d.pH larutan jika diencerkan dengan menambahkan 100 mL air
400 mL larutan 0,1 M direaksikan dengan 100 mL larutan 0,1 M. Tentukan: ()
a. pH larutan
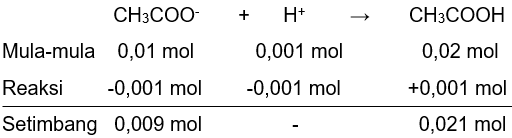
b. pH larutan jika ditambahkan 0,001 mol HCl
c. pH larutan jika ditambahkan 0,001 mol NaOH
d. pH larutan jika diencerkan dengan menambahkan 100 mL air
Iklan
IS
I. Solichah
Master Teacher
Jawaban terverifikasi
14
5.0 (1 rating)
Iklan
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2024 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia