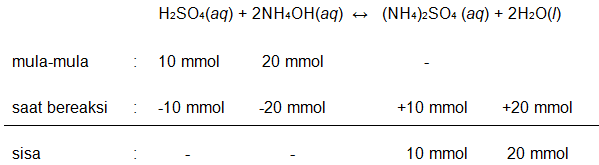Iklan
Iklan
Pertanyaan
100 mL H 2 SO 4 0,1 M direaksikan dengan 200 mL NH 4 OH 0,1 M ( K b = 1 × 1 0 − 5 ) . 1) K b = .... 2) [ H + ] = .... 3) [ OH − ] = .... 4) p H = .... 5) pO H = ....
100 mL 0,1 M direaksikan dengan 200 mL 0,1 M .
1) = ....
2) = ....
3) = ....
4) = ....
5) = ....
Iklan
IY
I. Yassa
Master Teacher
Jawaban terverifikasi
1
5.0 (1 rating)
NZ
NIKO ZADID DWI FIRNANDA
Makasih ❤️
Iklan
Iklan
Pertanyaan serupa
RUANGGURU HQ
Jl. Dr. Saharjo No.161, Manggarai Selatan, Tebet, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12860
Produk Ruangguru
Bantuan & Panduan
Hubungi Kami
©2024 Ruangguru. All Rights Reserved PT. Ruang Raya Indonesia